

Chinese General Practice ›› 2022, Vol. 25 ›› Issue (21): 2661-2669.DOI: 10.12114/j.issn.1007-9572.2022.0090
Special Issue: 骨健康最新文章合集
• Evidence-based Medicine • Previous Articles Next Articles
Received:2022-01-25
Revised:2022-03-18
Published:2022-07-20
Online:2022-05-19
Contact:
Hui GENG
About author:
通讯作者:
耿惠
作者简介:基金资助:
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.chinagp.net/EN/10.12114/j.issn.1007-9572.2022.0090
| 步骤 | 检索词 |
|---|---|
| #1 | Multiple Myeloma OR Multiple Myelomas OR Myelomas,Multiple OR Myeloma,Multiple OR Myeloma,Plasma-Cell OR Myeloma,Plasma Cell OR Myelomas,Plasma-Cell OR Plasma-Cell Myeloma OR Plasma-Cell Myelomas OR Myelomatosis OR Myelomatoses OR Plasma Cell Myeloma,Plasma OR Cell Myelomas,Plasma OR Myelomas,Plasma Cell OR Plasma Cell Myelomas OR Kahler Disease OR Disease,Kahler OR Myeloma-Multiple OR Myeloma Multiple OR Myeloma-Multiples |
| #2 | randomized controlled trial OR randomized OR placebo |
| #3 | Daratumumab OR humax-CD38 OR Darzalex |
| #4 | Isatuximab OR Sarclisa OR SAR650984 OR SAR |
| #5 | (#1 AND #2) AND #3 |
| #6 | (#1 AND #2) AND #4 |
Figure 1 Strategy for searching randomized controlled trials of CD38 monoclonal antibodies treating relapsed and refractory multiple myeloma in Web of Science
| 步骤 | 检索词 |
|---|---|
| #1 | Multiple Myeloma OR Multiple Myelomas OR Myelomas,Multiple OR Myeloma,Multiple OR Myeloma,Plasma-Cell OR Myeloma,Plasma Cell OR Myelomas,Plasma-Cell OR Plasma-Cell Myeloma OR Plasma-Cell Myelomas OR Myelomatosis OR Myelomatoses OR Plasma Cell Myeloma,Plasma OR Cell Myelomas,Plasma OR Myelomas,Plasma Cell OR Plasma Cell Myelomas OR Kahler Disease OR Disease,Kahler OR Myeloma-Multiple OR Myeloma Multiple OR Myeloma-Multiples |
| #2 | randomized controlled trial OR randomized OR placebo |
| #3 | Daratumumab OR humax-CD38 OR Darzalex |
| #4 | Isatuximab OR Sarclisa OR SAR650984 OR SAR |
| #5 | (#1 AND #2) AND #3 |
| #6 | (#1 AND #2) AND #4 |
| 第一作者 | 发表年份(年) | 既往治疗次数(次) | 随访时间(月) | 纳入例数 | 年龄(岁) | 干预措施 | ORR(%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 试验组 | 对照组 | 试验组 | 对照组 | 试验组 | 对照组 | |||||
| MARTIN[ | 2020 | 1~3 | 20.7 | 302 | 64(33,90) | IKd∶179 | Kd∶123 | 86.6 | 82.9 | |
| DIMOPOULOS[ | 2021 | ≥3 | — | 164 | 66(42,85) | 68(37,84) | Id∶55 | I∶109 | 43.6 | 23.9 |
| ATTAL[ | 2019 | ≥2 | 11.6 | 307 | 68(60,74) | 66(59,71) | IPd∶154 | Pd∶153 | 33.1 | 28.1 |
| DIMOPOULOS[ | 2020 | 1~3 | 17 | 466 | 64(57,70) | 65(59,71) | DKd∶312 | Kd∶154 | 84.3 | 74.7 |
| MATEOS[ | 2020 | ≥1 | 40 | 498 | 64(30,88) | 64(33,85) | DVd∶251 | Vd∶247 | 84.6 | 63.2 |
| BAHLIS[ | 2020 | ≥1 | 44.3 | 569 | 65(34,89) | 65(42,87) | DRd∶286 | Rd∶283 | 92.9 | 76.4 |
| DIMOPOULOS[ | 2021 | ≥1 | 16.9 | 304 | 67(42,86) | 68(35,90) | DPd∶151 | Pd∶153 | 68.9 | 46.4 |
| LU[ | 2021 | ≥1 | 8.2 | 211 | 61(28,79) | 61(43,82) | DVd∶141 | Vd∶70 | 82.5 | 58.6 |
Table 2 Basic characteristics of the included randomized controlled trials
| 第一作者 | 发表年份(年) | 既往治疗次数(次) | 随访时间(月) | 纳入例数 | 年龄(岁) | 干预措施 | ORR(%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 试验组 | 对照组 | 试验组 | 对照组 | 试验组 | 对照组 | |||||
| MARTIN[ | 2020 | 1~3 | 20.7 | 302 | 64(33,90) | IKd∶179 | Kd∶123 | 86.6 | 82.9 | |
| DIMOPOULOS[ | 2021 | ≥3 | — | 164 | 66(42,85) | 68(37,84) | Id∶55 | I∶109 | 43.6 | 23.9 |
| ATTAL[ | 2019 | ≥2 | 11.6 | 307 | 68(60,74) | 66(59,71) | IPd∶154 | Pd∶153 | 33.1 | 28.1 |
| DIMOPOULOS[ | 2020 | 1~3 | 17 | 466 | 64(57,70) | 65(59,71) | DKd∶312 | Kd∶154 | 84.3 | 74.7 |
| MATEOS[ | 2020 | ≥1 | 40 | 498 | 64(30,88) | 64(33,85) | DVd∶251 | Vd∶247 | 84.6 | 63.2 |
| BAHLIS[ | 2020 | ≥1 | 44.3 | 569 | 65(34,89) | 65(42,87) | DRd∶286 | Rd∶283 | 92.9 | 76.4 |
| DIMOPOULOS[ | 2021 | ≥1 | 16.9 | 304 | 67(42,86) | 68(35,90) | DPd∶151 | Pd∶153 | 68.9 | 46.4 |
| LU[ | 2021 | ≥1 | 8.2 | 211 | 61(28,79) | 61(43,82) | DVd∶141 | Vd∶70 | 82.5 | 58.6 |
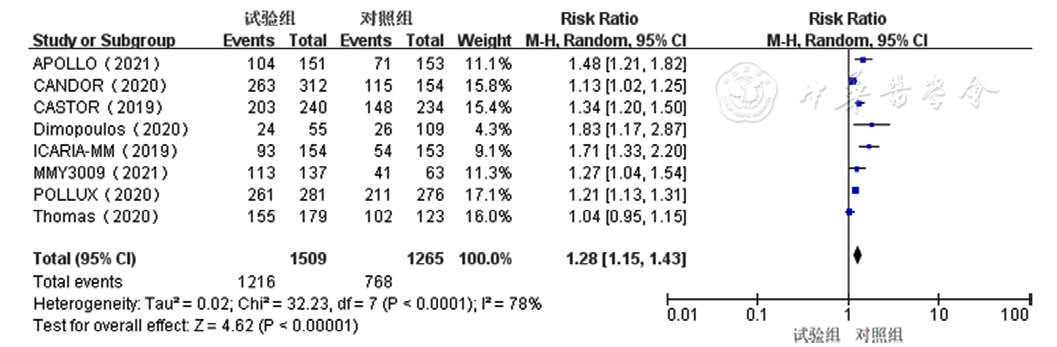
Figure 3 Forest plot of rates of overall response to treatment for relapsed and refractory multiple myeloma in the experimental group and control group
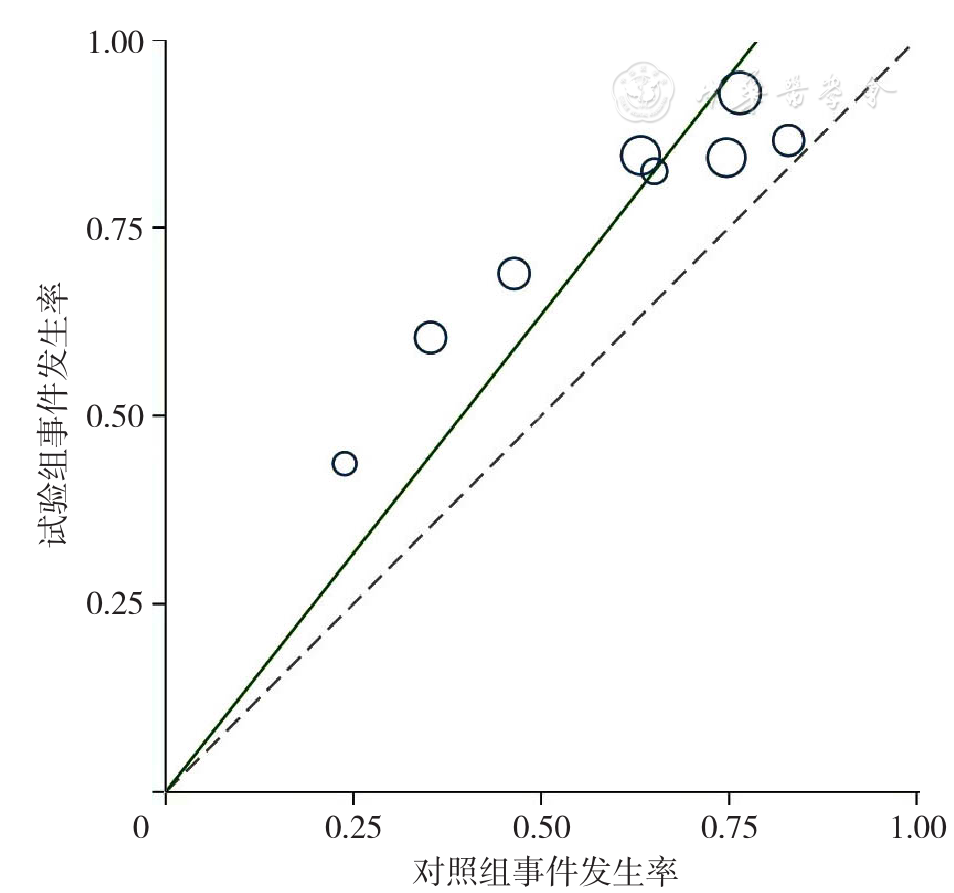
Figure 4 L'Abbe plotof rates of overall response to treatment for relapsed and refractory multiple myeloma in the experimental group and control group
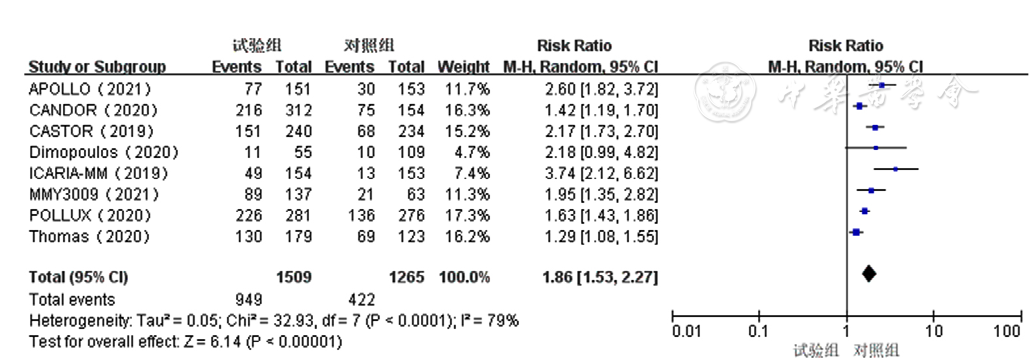
Figure 6 Forest plot assessing≥very good partial response to treatment for relapsed and refractory multiple myelomain the experimental group and control group
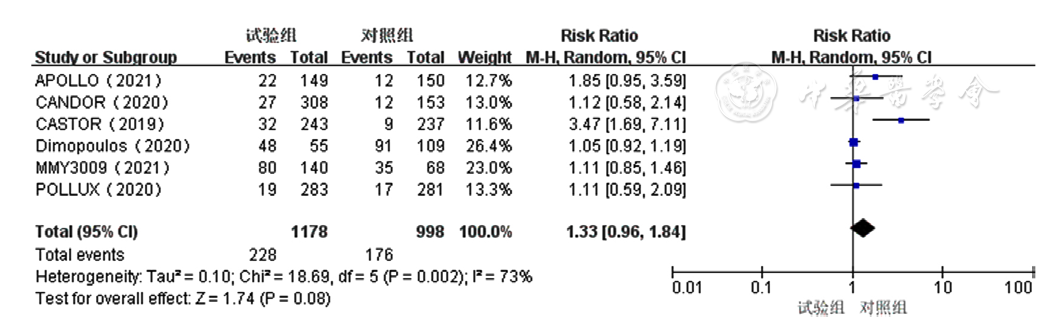
Figure 7 Forest plot assessing lymphocytopenia caused by treatment for relapsed and refractory multiple myeloma in the experimental group and control group
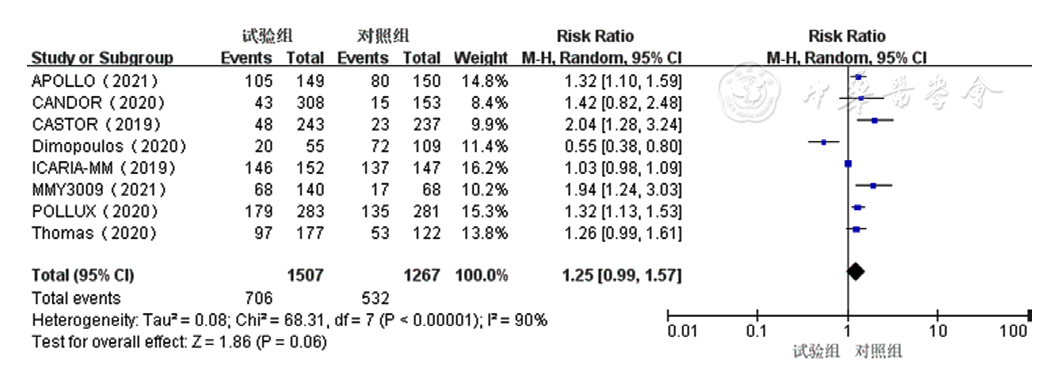
Figure 9 Forest plot assessing thrombocytopenia caused by treatment for relapsed and refractory multiple myeloma in the experimental group and control group
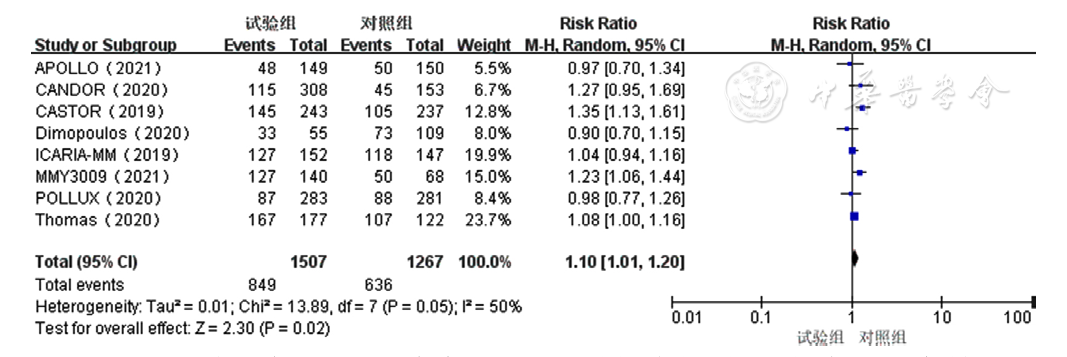
Figure 10 Forest plot assessing neutropenia caused by treatment for relapsed and refractory multiple myeloma in the experimental group and control group
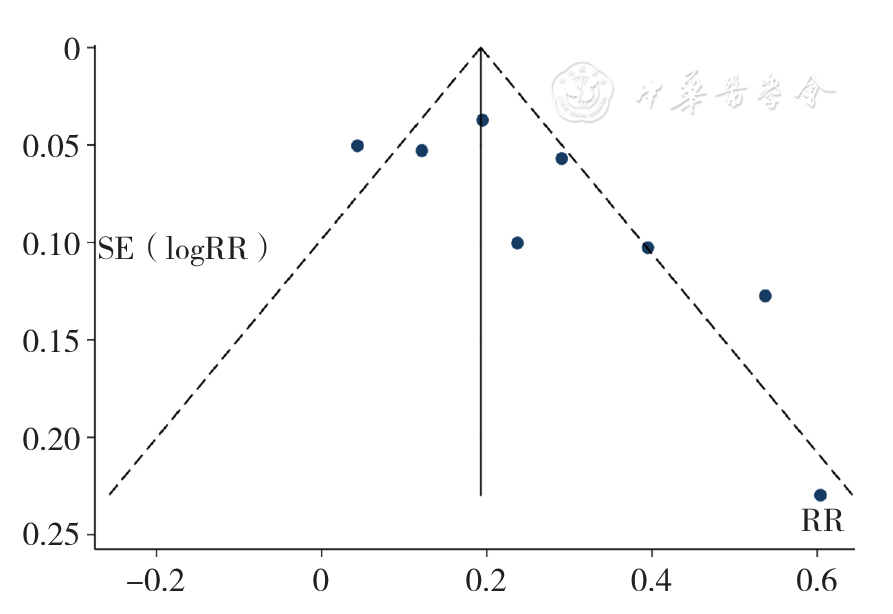
Figure 11 Funnel plot assessing publication bias of the rate of overall response to treatment for relapsed and refractory multiple myeloma between the experimental group and control group
| Std_Eff | 截距 | SE | t值 | P>|t| | 95%CI |
|---|---|---|---|---|---|
| slope | 0.035 | 0.081 | 0.43 | 0.679 | (-0.163,0.235) |
| bias | 2.807 | 1.305 | 2.15 | 0.075 | (-0.386,6.000) |
Table 4 Egger's test assessing potential publication bias
| Std_Eff | 截距 | SE | t值 | P>|t| | 95%CI |
|---|---|---|---|---|---|
| slope | 0.035 | 0.081 | 0.43 | 0.679 | (-0.163,0.235) |
| bias | 2.807 | 1.305 | 2.15 | 0.075 | (-0.386,6.000) |
| [1] |
|
| [2] |
《中国多发性骨髓瘤诊治指南(2020年修订)》发布[J].中华医学信息导报,2020,35(11):12.
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
王良哲,刘惠敏,侯健. 多发性骨髓瘤发病机制研究新进展[J]. 国际输血及血液学杂志,2007,30(4):319-322. DOI:10.3760/cma.j.issn.1673-419X.2007.04.008.
|
| [7] |
屈云,何俐,刘鸣. Cochrane系统评价的基本方法[J]. 中国临床康复,2003,7(4):532-533,536. DOI:10.3321/j.issn:1673-8225.2003.04.003.
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] | |
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [1] | YAO Yuzhong, MA Xiaojun, SONG Huan, ZHONG Yu. The Management Effect of Diabetes "1358 model" on Community Diabetes Patients Based on "Precision Management Combining General Care and Specialty Care" [J]. Chinese General Practice, 2023, 26(34): 4308-4314. |
| [2] | LI Qian, ZHANG Yunshu, YAN Baoping, WANG Jian, MA Yanjuan, WANG Yuan, QIN Yingjie, NA Long, REN Zhiyong, SUN Junwei, DENG Huaili, MA Hongjun, QU Xuehui, ZHOU Nan, SI Tianmei. Efficacy and Safety of Risperidone Microspheres for Injection (Ⅱ) in the Treatment of Patients with Acute Schizophrenia [J]. Chinese General Practice, 2023, 26(32): 4007-4012. |
| [3] | ZHANG Dongli, SHEN Chong, ZHANG Weichuan, CHEN Haibin, ZHAO Jianjun. Efficacy and Safety of Programmed Death-1/Programmed Death-1 Ligand Inhibitors in the Treatment of Renal Cell Cancer: a Meta-analysis [J]. Chinese General Practice, 2023, 26(30): 3815-3822. |
| [4] | LIU Ruifang, XU Fangxing, LIU Tongku, ZHOU Yujie, WU Xiaofan. Evaluation of the Efficacy and Safety of "Crowbar Effect" Technique to Facilitate Balloon Crossing Resistant Chronic Total Occlusions Lesions [J]. Chinese General Practice, 2023, 26(29): 3683-3688. |
| [5] | HUANG Dan, ZHANG Qihan, SONG Ge, WANG Qing, LI Yu, JI Xunming, WANG Yuan. Efficacy and Safety of Intermittent Hypoxic Training in the Prevention of Acute Hypoxic Injury [J]. Chinese General Practice, 2023, 26(29): 3640-3644. |
| [6] | HU Jingyi, HONG Jing, GUO Xiaodong, ZHANG Xiaohong, MO Ning, ZHOU Xiaocui, YU Qin, ZHOU Minhua, SUN Yan, NI Liu, SHI Xiaoli, SU Xiaoqing, LI Yuqian. Efficacy of Community-involved Hospice Care for Patients with Advanced Cancer: a Meta-analysis [J]. Chinese General Practice, 2023, 26(28): 3573-3584. |
| [7] | LU Bin, XIANG Chong, YUAN Xuesong, CAI Gaojun, WEI Wenfeng, YAN Yongmin. Effectiveness, Safety and Satisfaction of Distal Transradial Artery Approach in Cerebral Angiography [J]. Chinese General Practice, 2023, 26(27): 3378-3382. |
| [8] | LIU Minghao, WANG Pan, GAO Lijian, XU Shuqing, WANG Huanhuan, ZHAO Guangxian, CHEN Jue, QIAO Shubin, XU Bo, YUAN Jinqing. Feasibility, Safety and Timing of Secondary Percutaneous Coronary Intervention via Distal Transradial Artery Approach [J]. Chinese General Practice, 2023, 26(27): 3366-3372. |
| [9] | YAO Junjie, SHANG Qiangqiang, WANG Yufeng, LI Jiahui, LIU Chang, PANG Tingting. Wearable Inertial Sensors-based Efficacy Evaluation of Comprehensive Traditional Chinese Medicine Therapy for Lumbar Disc Herniation Due to Qi-stagnation and Blood-stasis [J]. Chinese General Practice, 2023, 26(27): 3450-3455. |
| [10] | ZHANG Yong, CAI Xiang, NING Feifei, LIANG Xiao, GUO Ning. Comparative Study of the Efficacy and Safety of Sacubitril/Valsartan and Dapagliflozin in the Treatment of Dilated Cardiomyopathy with Low Blood Pressure [J]. Chinese General Practice, 2023, 26(23): 2912-2917. |
| [11] | HE Manlan, YUAN Ping, HE Lei, CHEN Lu. Meta-analysis of Risk Factors for Urinary Tract Infection in Neurogenic Bladder [J]. Chinese General Practice, 2023, 26(21): 2659-2665. |
| [12] | GAO Yang, WANG Yunxia, GAO Chuanyu. Familial Hypercholesterolemia in 45-year-old and Younger Patients with Acute Coronary Syndrome: Clinical Characteristics and Influencing Factors of Blood Lipid Control Effect [J]. Chinese General Practice, 2023, 26(18): 2232-2237. |
| [13] | WANG Jun, WU Jiafei, WANG Yijing, ZHENG Boyue, WANG Yu, JIANG Chuanyan, LI Hui. Efficacy and Prognostic Effect of Daratumumab-based Chemotherapy Regimen in Multiple Myeloma: a Real-world Study [J]. Chinese General Practice, 2023, 26(18): 2256-2262. |
| [14] | CHEN Lulu, ZHANG Liping, LI Jingwen, DONG Wenjie, WU Xin'ai. Clinical Effect and Safety of PD-1 Inhibitors plus Fruquintinib as Later-line Treatment for Metastatic Colorectal Cancer [J]. Chinese General Practice, 2023, 26(18): 2262-2267. |
| [15] | CHANG Junpei, CHEN Lu, WU Tong, ZHAO Xiaoli, DUAN Fangfang, LIU Danna, KONG Tiandong. Occurrence and Treatment of Endocrinologic Adverse Reactions Associated with Immune Checkpoint Inhibitors: a Single-center Real-world Study [J]. Chinese General Practice, 2023, 26(17): 2095-2101. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||