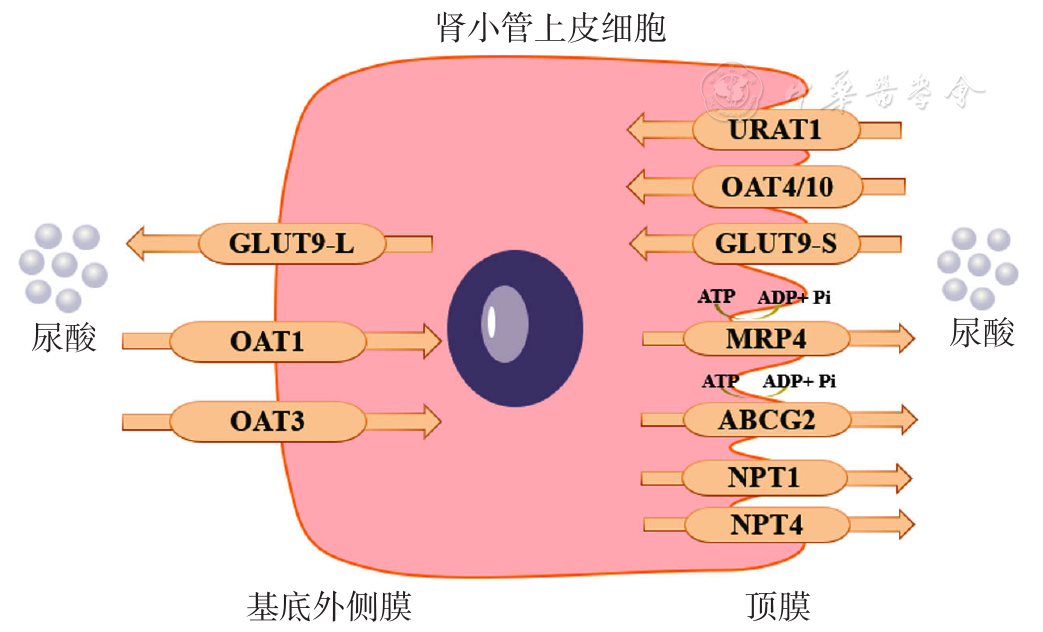
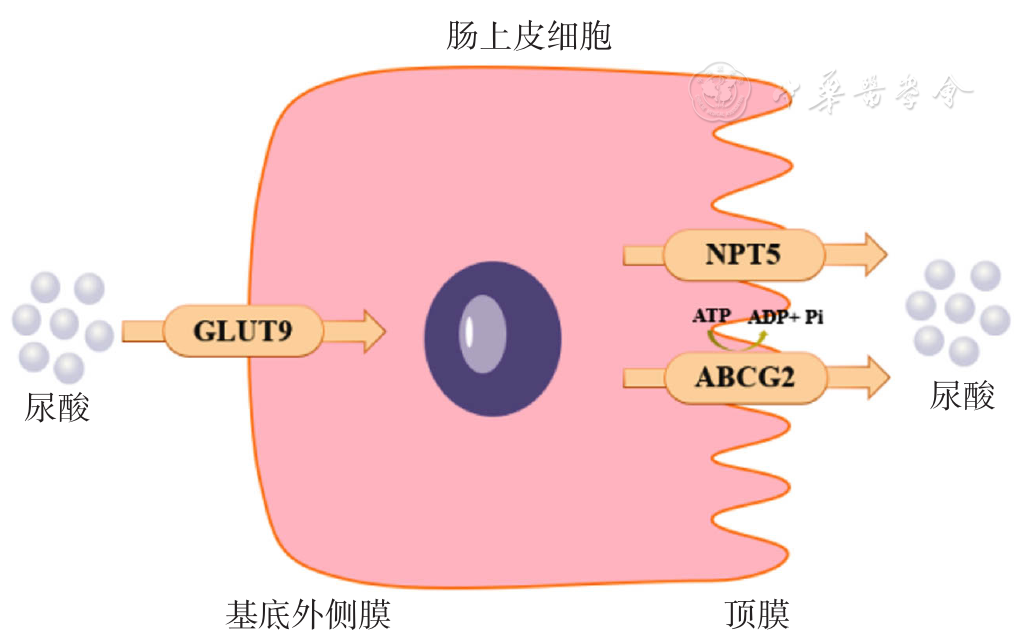
| [1] |
KANG D H, CHEN W. Uric acid and chronic kidney disease: new understanding of an old problem[J]. Semin Nephrol, 2011, 31(5):447-452. DOI: 10.1016/j.semnephrol.2011.08.009.
|
| [2] |
PETRESKI T, EKART R, HOJS R,et al. Hyperuricemia,the heart,and the kidneys - to treat or not to treat?[J]. Ren Fail, 2020, 42(1):978-986. DOI: 10.1080/0886022X.2020.1822185.
|
| [3] |
|
| [4] |
CHEN-XU M, YOKOSE C, RAI S K,et al. Contemporary prevalence of gout and hyperuricemia in the United States and decadal trends: the national health and nutrition examination survey,2007-2016[J]. Arthritis Rheumatol, 2019, 71(6):991-999. DOI: 10.1002/art.40807.
|
| [5] |
DEHLIN M, JACOBSSON L, RODDY E. Global epidemiology of gout: prevalence,incidence,treatment patterns and risk factors[J]. Nat Rev Rheumatol, 2020, 16(7):380-390. DOI: 10.1038/s41584-020-0441-1.
|
| [6] |
|
| [7] |
SOLTANI Z, RASHEED K, KAPUSTA D R,et al. Potential role of uric acid in metabolic syndrome,hypertension,kidney injury,and cardiovascular diseases: is it time for reappraisal?[J]. Curr Hypertens Rep, 2013, 15(3):175-181. DOI: 10.1007/s11906-013-0344-5.
|
| [8] |
ICHIDA K, MATSUO H, TAKADA T,et al. Decreased extra-renal urate excretion is a common cause of hyperuricemia[J]. Nat Commun, 2012, 3:764. DOI: 10.1038/ncomms1756.
|
| [9] |
WANG Z, CUI T, CI X Y,et al. The effect of polymorphism of uric acid transporters on uric acid transport[J]. J Nephrol, 2019, 32(2):177-187. DOI: 10.1007/s40620-018-0546-7.
|
| [10] |
RUIZ A, GAUTSCHI I, SCHILD L,et al. Human mutations in SLC2A9 (Glut9) affect transport capacity for urate[J]. Front Physiol, 2018, 9:476. DOI: 10.3389/fphys.2018.00476.
|
| [11] |
MANDAL A K, MERCADO A, FOSTER A,et al. Uricosuric targets of tranilast[J]. Pharmacol Res Perspect, 2017, 5(2):e00291. DOI: 10.1002/prp2.291.
|
| [12] |
ENOMOTO A, KIMURA H, CHAIROUNGDUA A,et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels[J]. Nature, 2002, 417(6887):447-452. DOI: 10.1038/nature742.
|
| [13] |
TAN P K, LIU S, GUNIC E,et al. Discovery and characterization of verinurad,a potent and specific inhibitor of URAT1 for the treatment of hyperuricemia and gout[J]. Sci Rep, 2017, 7(1):665. DOI: 10.1038/s41598-017-00706-7.
|
| [14] |
HIGASHINO T, MATSUO H, SAKIYAMA M,et al. Common variant of PDZ domain containing 1 (PDZK1) gene is associated with gout susceptibility: a replication study and meta-analysis in Japanese population[J]. Drug Metab Pharmacokinet, 2016, 31(6):464-466. DOI: 10.1016/j.dmpk.2016.07.004.
|
| [15] |
MISAWA K, HASEGAWA T, MISHIMA E,et al. Contribution of rare variants of the SLC22A12 gene to the missing heritability of serum urate levels[J]. Genetics, 2020, 214(4):1079-1090. DOI: 10.1534/genetics.119.303006.
|
| [16] |
SAKIYAMA M, MATSUO H, SHIMIZU S,et al. The effects of URAT1/SLC22A12 nonfunctional variants,R90H and W258X,on serum uric acid levels and gout/hyperuricemia progression[J]. Sci Rep, 2016, 6:20148. DOI: 10.1038/srep20148.
|
| [17] |
SHIN H J, TAKEDA M, ENOMOTO A,et al. Interactions of urate transporter URAT1 in human kidney with uricosuric drugs[J]. Nephrology (Carlton), 2011, 16(2):156-162. DOI: 10.1111/j.1440-1797.2010.01368.x.
|
| [18] |
XU L, LU L L, GAO J D. Traditional Chinese herbal medicine plays a role in the liver,kidney,and intestine to ameliorate hyperuricemia according to experimental studies[J]. Evid Based Complement Alternat Med, 2021, 2021:4618352. DOI: 10.1155/2021/4618352.
|
| [19] |
NOVIKOV A, FU Y L, HUANG W,et al. SGLT2 inhibition and renal urate excretion: role of luminal glucose,GLUT9,and URAT1[J]. Am J Physiol Renal Physiol, 2019, 316(1):F173-185. DOI: 10.1152/ajprenal.00462.2018.
|
| [20] |
|
| [21] |
DINOUR D, GRAY N K, CAMPBELL S,et al. Homozygous SLC2A9 mutations cause severe renal hypouricemia[J]. J Am Soc Nephrol, 2010, 21(1):64-72. DOI: 10.1681/ASN.2009040406.
|
| [22] |
MANCIKOVA A, KRYLOV V, HURBA O,et al. Functional analysis of novel allelic variants in URAT1 and GLUT9 causing renal hypouricemia type 1 and 2[J]. Clin Exp Nephrol, 2016, 20(4):578-584. DOI: 10.1007/s10157-015-1186-z.
|
| [23] |
XU X X, LI C H, ZHOU P,et al. Uric acid transporters hiding in the intestine[J]. Pharm Biol, 2016, 54(12):3151-3155. DOI: 10.1080/13880209.2016.1195847.
|
| [24] |
HAGOS Y, STEIN D, UGELE B,et al. Human renal organic anion transporter 4 operates as an asymmetric urate transporter[J]. J Am Soc Nephrol, 2007, 18(2):430-439. DOI: 10.1681/ASN.2006040415.
|
| [25] |
BAHN A, HAGOS Y, REUTER S,et al. Identification of a new urate and high affinity nicotinate transporter,hOAT10 (SLC22A13)[J]. J Biol Chem, 2008, 283(24):16332-16341. DOI: 10.1074/jbc.M800737200.
|
| [26] |
SAKIYAMA M, MATSUO H, SHIMIZU S,et al. A common variant of organic anion transporter 4 (OAT4/SLC22A11) gene is associated with renal underexcretion type gout[J]. Drug Metab Pharmacokinet, 2014, 29(2):208-210. DOI: 10.2133/dmpk.dmpk-13-nt-070.
|
| [27] |
CHUNG S, KIM G H. Urate transporters in the kidney: what clinicians need to know[J]. Electrolyte Blood Press, 2021, 19(1):1-9. DOI: 10.5049/EBP.2021.19.1.1.
|
| [28] |
MATSUO H, TAKADA T, ICHIDA K,et al. Common defects of ABCG2,a high-capacity urate exporter,cause gout: a function-based genetic analysis in a Japanese population[J]. Sci Transl Med, 2009, 1(5):5ra11. DOI: 10.1126/scitranslmed.3000237.
|
| [29] |
DOYLE L A, YANG W, ABRUZZO L V,et al. A multidrug resistance transporter from human MCF-7 breast cancer cells[J]. Proc Natl Acad Sci USA, 1998, 95(26):15665-15670. DOI: 10.1073/pnas.95.26.15665.
|
| [30] |
|
| [31] |
TOYODA Y, MAN ÍKOVÁ A, KRYLOV V,et al. Functional characterization of clinically-relevant rare variants in ABCG2 identified in a gout and hyperuricemia cohort[J]. Cells, 2019, 8(4):363. DOI: 10.3390/cells8040363.
|
| [32] |
FUJITA K, YAMADA H, IIJIMA M,et al. Electrochemical analysis of uric acid excretion to the intestinal lumen: effect of serum uric acid-lowering drugs and 5/6 nephrectomy on intestinal uric acid levels[J]. PLoS One, 2019, 14(12):e0226918. DOI: 10.1371/journal.pone.0226918.
|
| [33] |
KOMORI H,YAMADA K,TAMAI I. Hyperuricemia enhances intracellular urate accumulation via down-regulation of cell-surface BCRP/ABCG2 expression in vascular endothelial cells[J]. Biochim Biophys Acta Biomembr,2018,1860(5):973-980.
|
| [34] |
MATSUO H, ICHIDA K, TAKADA T,et al. Common dysfunctional variants in ABCG2 are a major cause of early-onset gout[J]. Sci Rep, 2013, 3:2014. DOI: 10.1038/srep02014.
|
| [35] |
MATSUO H, TSUNODA T, OOYAMA K,et al. Hyperuricemia in acute gastroenteritis is caused by decreased urate excretion via ABCG2[J]. Sci Rep, 2016, 6:31003. DOI: 10.1038/srep31003.
|
| [36] |
WOODWARD O M, KÖTTGEN A, CORESH J,et al. Identification of a urate transporter,ABCG2,with a common functional polymorphism causing gout[J]. Proc Natl Acad Sci USA, 2009, 106(25):10338-10342. DOI: 10.1073/pnas.0901249106.
|
| [37] |
NIGAM S K. The SLC22 transporter family: a paradigm for the impact of drug transporters on metabolic pathways,signaling,and disease[J]. Annu Rev Pharmacol Toxicol, 2018, 58:663-687. DOI: 10.1146/annurev-pharmtox-010617-052713.
|
| [38] |
|
| [39] |
NIGAM S K, BUSH K T, MARTOVETSKY G,et al. The organic anion transporter (OAT) family: a systems biology perspective[J]. Physiol Rev, 2015, 95(1):83-123. DOI: 10.1152/physrev.00025.2013.
|
| [40] |
LIU H C, JAMSHIDI N, CHEN Y C,et al. An organic anion transporter 1 (OAT1)-centered metabolic network[J]. J Biol Chem, 2016, 291(37):19474-19486. DOI: 10.1074/jbc.M116.745216.
|
| [41] |
YONG T Q, CHEN S D, XIE Y Z,et al. Hypouricemic effects of Armillaria mellea on hyperuricemic mice regulated through OAT1 and CNT2[J]. Am J Chin Med, 2018, 46(3):585-599. DOI: 10.1142/S0192415X18500301.
|
| [42] |
FAN Y Z, LIANG Z X, ZHANG J H,et al. Oral proteasomal inhibitors ixazomib,oprozomib,and delanzomib upregulate the function of organic anion transporter 3 (OAT3): implications in OAT3-mediated drug-drug interactions[J]. Pharmaceutics, 2021, 13(3):314. DOI: 10.3390/pharmaceutics13030314.
|
| [43] |
DRAGOJEVI J, MIHALJEVI I, POPOVI M,et al. Zebrafish (Danio rerio) Oat1 and Oat3 transporters and their interaction with physiological compounds[J]. Comp Biochem Physiol B Biochem Mol Biol, 2019, 236:110309. DOI: 10.1016/j.cbpb.2019.110309.
|
| [44] |
TRUONG D M, KALER G, KHANDELWAL A,et al. Multi-level analysis of organic anion transporters 1,3,and 6 reveals major differences in structural determinants of antiviral discrimination[J]. J Biol Chem, 2008, 283(13):8654-8663. DOI: 10.1074/jbc.M708615200.
|
| [45] |
WU W, BUSH K T, NIGAM S K. Key role for the organic anion transporters,OAT1 and OAT3,in the in vivo handling of uremic toxins and solutes[J]. Sci Rep, 2017, 7(1):4939. DOI: 10.1038/s41598-017-04949-2.
|
| [46] |
OTANI N, OUCHI M, HAYASHI K,et al. Roles of organic anion transporters (OATs) in renal proximal tubules and their localization[J]. Anat Sci Int, 2017, 92(2):200-206. DOI: 10.1007/s12565-016-0369-3.
|
| [47] |
SUN H L, WU Y W, BIAN H G,et al. Function of uric acid transporters and their inhibitors in hyperuricaemia[J]. Front Pharmacol, 2021, 12: 667753. DOI: 10.3389/fphar.2021.667753.
|
| [48] |
VAN AUBEL R A M H, SMEETS P H E, PETERS J G P,et al. The MRP4/ABCC4 gene encodes a novel apical organic anion transporter in human kidney proximal tubules: putative efflux pump for urinary cAMP and cGMP[J]. J Am Soc Nephrol, 2002, 13(3):595-603. DOI: 10.1681/ASN.V133595.
|
| [49] |
DONG Z, ZHOU J R, JIANG S,et al. Effects of multiple genetic loci on the pathogenesis from serum urate to gout[J]. Sci Rep, 2017, 7:43614. DOI: 10.1038/srep43614.
|
| [50] |
TANNER C, BOOCOCK J, STAHL E A,et al. Population-specific resequencing associates the ATP-binding cassette subfamily C member 4 gene with gout in New Zealand māori and Pacific men[J]. Arthritis Rheumatol, 2017, 69(7):1461-1469. DOI: 10.1002/art.40110.
|
| [51] |
SLOT A J, MOLINSKI S V, COLE S P C. Mammalian multidrug-resistance proteins (MRPs)[J]. Essays Biochem, 2011, 50(1):179-207. DOI: 10.1042/bse0500179.
|
| [52] |
DING X D, LI M M, PENG C L,et al. Uric acid transporters BCRP and MRP4 involved in chickens uric acid excretion[J]. BMC Vet Res, 2019, 15(1):180. DOI: 10.1186/s12917-019-1886-9.
|
| [53] |
|
| [54] |
CHIBA T, MATSUO H, KAWAMURA Y,et al. NPT1/SLC17A1 is a renal urate exporter in humans and its common gain-of-function variant decreases the risk of renal underexcretion gout[J]. Arthritis Rheumatol, 2015, 67(1):281-287. DOI: 10.1002/art.38884.
|
| [55] |
JUTABHA P, ANZAI N, WEMPE M F,et al. Apical voltage-driven urate efflux transporter NPT4 in renal proximal tubule[J]. Nucleosides Nucleotides Nucleic Acids, 2011, 30(12):1302-1311. DOI: 10.1080/15257770.2011.616564.
|
| [56] |
SUN H L, WU Y W, BIAN H G,et al. Function of uric acid transporters and their inhibitors in hyperuricaemia[J]. Front Pharmacol, 2021, 12:667753. DOI: 10.3389/fphar.2021.667753.
|
| [57] |
JUTABHA P, ANZAI N, KITAMURA K,et al. Human sodium phosphate transporter 4 (hNPT4/SLC17A3) as a common renal secretory pathway for drugs and urate[J]. J Biol Chem, 2010, 285(45):35123-35132. DOI: 10.1074/jbc.M110.121301.
|
| [58] |
RISTIC B, SIKDER M O F, BHUTIA Y D,et al. Pharmacologic inducers of the uric acid exporter ABCG2 as potential drugs for treatment of gouty arthritis[J]. J Asian J Pharm Sci, 2020, 15(2):173-180. DOI: 10.1016/j.ajps.2019.10.002.
|
| [59] |
LAU H S, WISEMAN R F. Effect of uric acid on riboflavine production by intestinal coliform bacteria[J]. J Bacteriol, 1964, 87(2):337-340. DOI: 10.1128/jb.87.2.337-340.1964.
|
| [60] |
YAMADA N, IWAMOTO C, KANO H,et al. Evaluation of purine utilization by Lactobacillus gasseri strains with potential to decrease the absorption of food-derived purines in the human intestine[J]. Nucleosides Nucleotides Nucleic Acids, 2016, 35(10-12):670-676. DOI: 10.1080/15257770.2015.1125000.
|
| [61] |
WU J W, WEI Z H, CHENG P,et al. Rhein modulates host purine metabolism in intestine through gut microbiota and ameliorates experimental colitis[J]. Theranostics, 2020, 10(23):10665-10679. DOI: 10.7150/thno.43528.
|
| [62] |
DEBOSCH B J, KLUTH O, FUJIWARA H,et al. Early-onset metabolic syndrome in mice lacking the intestinal uric acid transporter SLC2A9[J]. Nat Commun, 2014, 5:4642. DOI: 10.1038/ncomms5642.
|
| [63] |
LI Q R, LIN H, NIU Y F,et al. Mangiferin promotes intestinal elimination of uric acid by modulating intestinal transporters[J]. Eur J Pharmacol, 2020, 888:173490. DOI: 10.1016/j.ejphar.2020.173490.
|
| [64] |
HALPERIN KUHNS V L, WOODWARD O M. Urate transport in health and disease[J]. Best Pract Res Clin Rheumatol, 2021, 35(4):101717. DOI: 10.1016/j.berh.2021.101717.
|
| [65] |
|
| [66] |
SARKADI B, OZVEGY-LACZKA C, NÉMET K,et al. ABCG2 —a transporter for all seasons[J]. FEBS Lett, 2004, 567(1):116-120. DOI: 10.1016/j.febslet.2004.03.123.
|
| [67] |
CHEN M, LU X Y, LU C,et al. Soluble uric acid increases PDZK1 and ABCG2 expression in human intestinal cell lines via the TLR4-NLRP3 inflammasome and PI3K/Akt signaling pathway[J]. Arthritis Res Ther, 2018, 20(1):20. DOI: 10.1186/s13075-018-1512-4.
|
| [68] |
MATSUO H, TSUNODA T, OOYAMA K,et al. Hyperuricemia in acute gastroenteritis is caused by decreased urate excretion via ABCG2[J]. Sci Rep, 2016, 6:31003. DOI: 10.1038/srep31003.
|
| [69] |
ICHIDA K, MATSUO H, TAKADA T,et al. Decreased extra-renal urate excretion is a common cause of hyperuricemia[J]. Nat Commun, 2012, 3:764. DOI: 10.1038/ncomms1756.
|
| [70] |
YANO H, TAMURA Y, KOBAYASHI K,et al. Uric acid transporter ABCG2 is increased in the intestine of the 5/6 nephrectomy rat model of chronic kidney disease[J]. Clin Exp Nephrol, 2014, 18(1):50-55. DOI: 10.1007/s10157-013-0806-8.
|
| [71] |
BHATNAGAR V, RICHARD E L, WU W,et al. Analysis of ABCG2 and other urate transporters in uric acid homeostasis in chronic kidney disease: potential role of remote sensing and signaling[J]. Clin Kidney J, 2016, 9(3):444-453. DOI: 10.1093/ckj/sfw010.
|
| [72] |
MATSUO H, ICHIDA K, TAKADA T,et al. Common dysfunctional variants in ABCG2 are a major cause of early-onset gout[J]. Sci Rep, 2013, 3:2014. DOI: 10.1038/srep02014.
|
| [73] |
SHIBUI A, TSUNODA T, SEKI N,et al. Isolation and chromosomal mapping of a novel human gene showing homology to Na +/PO 4 cotransporter[J]. J Hum Genet, 1999, 44(3):190-192. DOI: 10.1007/s100380050140.
|
| [74] |
Togawa, Natsuko,et al. A Na +-phosphate cotransporter homologue (SLC17A4 protein) is an intestinal organic anion exporter[J]. Am J Physiol Cell Physiol, 2012, 302(11):C1652-1660. DOI: 10.1152/ajpcell.00015.2012.
|
 )
)
 )
)